Answer:
d = 0.0735 g/mL
Step-by-step explanation:
Given that,
The accepted value of density of a penny is 7.2 g/mL
Mass of the penny, m = 2.5 g
Volume,
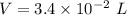
Since, 1 L = 1000 mL
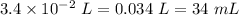
The density of the experiment value is given by :
Density = mass/volume
So,
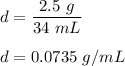
Hence, the experimental value for the density of the penny is 0.0735 g/mL.