Answer:
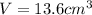
Step-by-step explanation:
Hello,
In this case, given the reaction in which the acetic acid reacts with strontium hydroxide to yield water and strontium acetate:
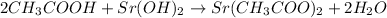
The first step here is to compute the moles of strontium hydroxide that are reacting given its volume in liters (0.250 L) and concentration:
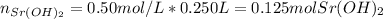
Next, considering the 1:2 mole ratio between the strontium hydroxide and the acetic acid (molar mass = 60 g/mol) we compute the grams of acid that are consumed:
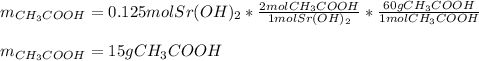
Then, by using the density of the acetic acid, we compute the volume:
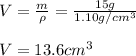
Best regards.