Given :
The heat of vaporization for water is 2330 J/g.
To Find :
How many grams of water can be vaporized with 6500 J of energy.
Solution :
It is given that heat of vaporization for water is 2330 J/g.
Means 2300 J of heat is required to vaporize 1 g of water .
Let , n grams of water is produced by 6500 J of energy .
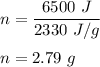
Therefore, 2.79 g of water will vaporize by 6500 J of energy .
Hence, this is the required solution .