Answer:
Step-by-step explanation:
The relation between average velocity of a gas molecules and its molecular mass is as follows .
average velocity v
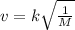 where k is a constant .
where k is a constant .
If v₁ and v₂ be the velocities corresponding to molecular masses 349 and 352 .
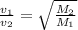
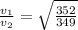
= 1.004