Answer:

Step-by-step explanation:
Density is found by dividing the mass by the volume.
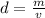
The mass of the liquid is 12.7 grams.
We know that 15 mL of this liquid was added to a 50 mL graduated cylinder. Therefore, the volume is 15 mL. The 50 mL is not relevant, it only tells us about the graduated cylinder.
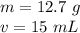
Substitute the values into the formula.
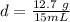
Divide.
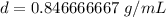
Round to the nearest hundredth. The 6 in the tenth place tells us to round the 4 to a 5.
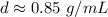
The density of the liquid is about 0.85 grams per milliliter and choice A is correct.