Answer:
C) 6.95 M.
Step-by-step explanation:
Hello,
In this case, considering that the molarity is computed via the ratio of the moles of solute to the volume of the solution in liters:
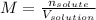
Since the volume here is 95.0 mL or also 0.0950 L, the molarity turns out:
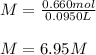
Therefore, answer is C) 6.95M.
Best regards.