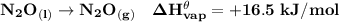Answer:
The standard heat of vaporization of nitrous oxide is 16.5 kJ/mol
Step-by-step explanation:
The standard heat of vaporization
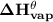 , occurs as the result of a change in heat when 1 mole of a liquid is vaporized. The standard heat of vaporization is always positive i.e endothermic. In the given question, we are asked to determine what the standard heat of vaporization of nitrous oxide.
, occurs as the result of a change in heat when 1 mole of a liquid is vaporized. The standard heat of vaporization is always positive i.e endothermic. In the given question, we are asked to determine what the standard heat of vaporization of nitrous oxide.
i.e