Given :
Concentration of product [A] = 0.371 M .
Rate constant ,
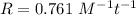 .
.
To Find :
The rate for the reaction .
Solution :
We know , for second order reaction , rate is given by :
![r=k[A]^2\\\\r=0.761* 0.371^2\ M/t\\\\r=0.10\ M/t](https://img.qammunity.org/2021/formulas/chemistry/college/7rvow6c1xg81w70ymccc66w7ennfs14ycq.png)
Therefore , the rate for the second order reaction is 0.1 M/t .
Hence , this is the required solution .