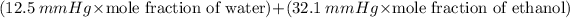Answer:
Step-by-step explanation:
Since we are not given the mole fraction of ethanol and water; we will solve this theoretically.
Using Raoult's Law:
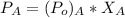
For water:
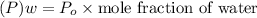
where
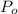 of water = 12.5 mmHg
of water = 12.5 mmHg
Then, the vapor pressure of water:
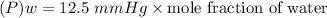
For ethanol:
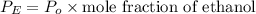
and the
 of ethanol = 32.1 mmHg
of ethanol = 32.1 mmHg
Then, the vapor pressure of ethanol:
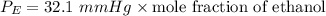
The total vapor pressure
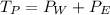
The total vapor pressure =