Given :
Volume of
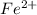 , V = 132 mL .
, V = 132 mL .
Molarity of
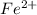 , M = 0.1782 M .
, M = 0.1782 M .
To Find :
How many milliliters of 0.2937 M
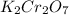 are required to titrate 132.0 mL of 0.1782 M
are required to titrate 132.0 mL of 0.1782 M
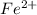 solution .
solution .
Solution :
Moles of
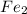 :
:
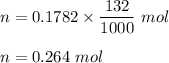
Now , 1 mole of
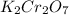 reacts with 6 mole of
reacts with 6 mole of
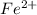 .
.
So , moles of
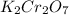 required is :
required is :
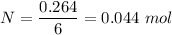
Volume required is :
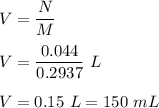
Hence , this is the required solution .