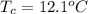Answer:
The value is
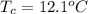
Step-by-step explanation:
From the question we are told that
The mass of the ice cube is
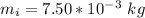
The temperature of the ice cube is
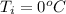
The mass of the copper cube is
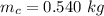
The final temperature of both substance is
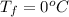
Generally form the law of thermal energy conservation,
The heat lost by the copper cube = heat gained by the ice cube
Generally the heat lost by the copper cube is mathematically represented as
![Q = m_c * c_c * [T_c - T_f ]](https://img.qammunity.org/2021/formulas/physics/college/wruqc81xuu0rjddoijjuu0nmyycugjyspa.png)
The specific heat of copper is
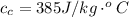
Generally the heat gained by the ice cube is mathematically represented as
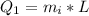
Here L is the latent heat of fusion of the ice with value
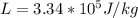
So
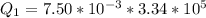
=>
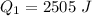
So
![2505 = 0.540 * 385 * [T_c - 0 ]](https://img.qammunity.org/2021/formulas/physics/college/a76ov9lo6uxd702u3d66jieh0hon23103u.png)
=>