Answer:
a.
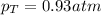 .
.
b.
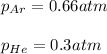
c.
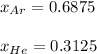
Step-by-step explanation:
Hello,
In this case, considering that the valve is opened, we can use the Boyle's law in order to compute the final pressure of argon by considering its initial pressure and volume and a final volume of 5.0 L:
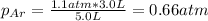
And the final pressure of helium:
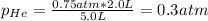
Which actually are the partial pressure of both of them, it means that the total pressure is:
Finally, the mole fraction of each gas is computed by considering the Dalton's law:
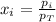

Best regards.