Answer:
The final temperature of the water is 23.92°C.
Step-by-step explanation:
Given that,
A heater in a hot water tank gives off 20,000 kJ per hour.
For 1 hour or 60 minutes, heat is 20,000 kJ
For 90 minutes,
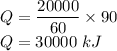
Mass, m = 450 kg or 450000 grams
Initial temperature,
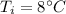
We need to find the final temperature of the water. We know that the specific heat capacity of water is 4.186 J/g- °C
Heat is given by :
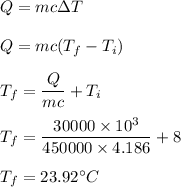
Hence, the final temperature of the water is 23.92°C.