Answer:
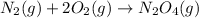
Step-by-step explanation:
Hello,
In this case, considering the required product which is dinitrogen tetroxide whose molecular formula is N₂O₄, by considering its formation reaction which is starting by both gaseous nitrogen and oxygen as shown below:
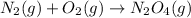
Nevertheless, it should be balance since four oxygen atoms are present at the right side, thus, we obtain:
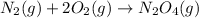
Best regards.