Given:
Two electrons are added to each of six atoms in a group.
To find:
The expression for the net change in charge.
Solution:
Charge of atoms = Number protons - Number of electrons.
Let number of atoms be x.
6 atoms = 2 electrons added
1 atom =
 electrons added
electrons added
1 atom =
 electrons added
electrons added
x atoms =
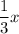 electrons added
electrons added
Therefore, the net change in charge is represented by the expression
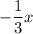 because we subtract number of electrons to find atom charge, where, x is the number of atoms.
because we subtract number of electrons to find atom charge, where, x is the number of atoms.
6 atoms = 2 protons removed
1 atom =
 protons removed
protons removed
1 atom =
 protons removed
protons removed
x atoms =
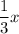 protons removed
protons removed
Therefore, the net change in charge is represented by the expression
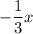 , where, x is the number of atoms.
, where, x is the number of atoms.