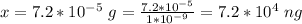Answer:
The mass of
 present is
present is
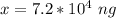
Step-by-step explanation:
From the question we are told that
The diameter of the spot is
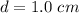
The volume of the solution present is
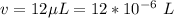
The mass of
 ions in one liter of solution is
ions in one liter of solution is
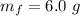
Generally the mass of
 ions present is v is mathematically represented as
ions present is v is mathematically represented as
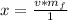
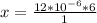
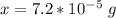
Converting to nanograms
We have