Answer:
Five molecules of oxygen.
Step-by-step explanation:
Hello,
In this case, considering the given chemical reaction, we must write a five before the oxygen in order to equal the number of oxygen atoms at both the right and left side (ten) so phosphorous remain the same (4):
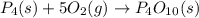
It means that five molecules of oxygen (O₂) are needed to form one molecule of tetraphosphorous decaoxide (P₄O₁₀).
Best regards.