Given :
2NOBr(g) - -> 2NO(g) + Br2(g)
Initial pressure of NOBr , 1 atm .
At equilibrium, the partial pressure of NOBr is 0.82 atm.
To Find :
The equilibrium constant for the reaction .
Solution :
2NOBr(g) - -> 2NO(g) + Br2(g)
t=0 s 1 atm 0 0
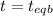 1( 1-2x) 2x x
1( 1-2x) 2x x
So ,
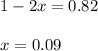
At equilibrium :
![K_(eq)=([NO]^2[br_2])/([NOBr]^2)\\\\K_(eq)=(0.18^2* 0.9)/(0.82^2)\\\\K_(eq)=0.043\ atm](https://img.qammunity.org/2021/formulas/chemistry/high-school/h8z8y3wca5bqmsrkye7rdymuroazpitmmh.png)
Hence , this is the required solution .