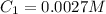Complete Question
In a laboratory experiment, you are asked to determine the molar concentration of a solution of an unknown compound, X. The solution diluted in with water (200 µL of X + 800 µL of H2O) has an absorbance at 425 nm of 0.8 and a molar extinction coefficient of 1.5 x103 M-1cm-1 at 425 nm. What is the molar concentration of the original solution of X? (1 cm cuvette)
Answer:
The original concentration is
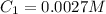
Step-by-step explanation:
From the question we are told that
The original volume of solution X is
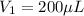
The volume of solution X after dilution is
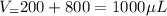
The absorbance is
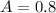
The molar extinction coefficient is
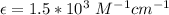
Generally from Beer's law

Here
L is the path length with a value of 1 cm
C_2 is the concentration of the solution at the given absorbance
=>
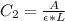
=>
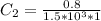
=>
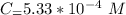
Generally we have that
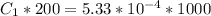
=>