Answer:
-57792 C
Step-by-step explanation:
Recall that in one mole of a substance there are an Avogadro number of molecules. That is:
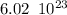 molecules
molecules
therefore, in 1.2 g, there would be ten times less molecules than in the 12.0 g mole, that is:
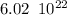 molecules.
molecules.
Now, each C there are 6 negative charges, then, the number of electrons will be six times the number of C molecules, that is:
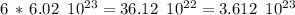 electrons.
electrons.
Now we multiply this number times the charge of an electron
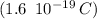 , and we obtained the total negative charge of the carbon sample:
, and we obtained the total negative charge of the carbon sample:
Total negative charge is:
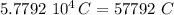
therefore the total negative charge is: -57792 C