Answer:
a
No
b
100 mm Hg
Step-by-step explanation:
From the question we are told that
The vapor pressure of CHCl3, is
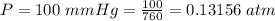
The temperature of CHCl3 is
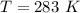
The volume of the container is
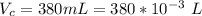
The temperature of the container is
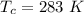
The mass of CHCl3 is m = 0.380 g
Generally the number of moles of CHCl3 present before evaporation started is mathematically represented as
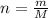
Here M is the molar mass of CHCl3 with the value
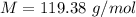
=>
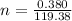
=>
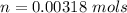
Generally the number of moles of CHCl3 gas that evaporated is mathematically represented as
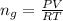
Here R is the gas constant with value
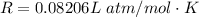
So
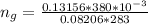
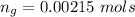
Given that the number of moles of CHCl3 evaporated is less than the number of moles of CHCl3 initially present , then it mean s that not all the liquid evaporated
At equilibrium the temperature of CHCl3 will be equal to the pressure of air so the pressure at equilibrium is 100 mmHg