Answer:
Change in free energy (ΔG) = 1.43 kcal/mol
Step-by-step explanation:
Free energy is the energy released by a diffusing molecule moving up or down a concentration gradient, across a membrane.
The formula for free energy change import into a membrane is given by the formula :
![\triangle G = \triangle G^0 + R * T * In([X]_(inside))/([X]_(outsdie))](https://img.qammunity.org/2021/formulas/biology/college/qz2ohsztfkps77agruz75oyg3nuq6a31oj.png)
where:
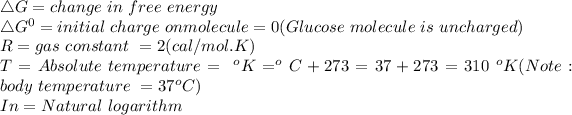
![[X]_(inside) = concentration\ of\ glucose\ inside\ the\ cell = 0.5mM\\ {[X]}_(outside) = concentration\ of\ glucose\ outside\ the\ cell = 5mM](https://img.qammunity.org/2021/formulas/biology/college/ysdwlcf7yqbufyu16iircv9mdql6euac2a.png)
![\triangle G = 0 + (2) * (310) * In([0.5])/([5]) \\\triangle G = 620 * In (0.1)\\\triangle G = 620 * (-2.3)\\\triangle G = -1426\ cal/mol\\\triangle G = 1.43\ kcal/mol](https://img.qammunity.org/2021/formulas/biology/college/elakzogze62b3vf8dukfk78uh4wwtkk8pe.png)