Answer:
MM/n will be less than the theoretical value
Step-by-step explanation:
Provided that
The plating efficiency is proportional to the element weight
i.e.
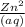
That represents
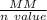
So,
MM by N value is
= 65.39 g /mol ÷ 2
= 32.70 g/mol
Also, the experimental plating value is 0.55 that represents that it is much lesser than the normal value
So it would be concluded that the MM by N would be lower than the theoretical value