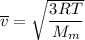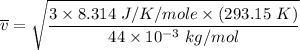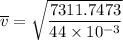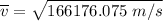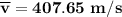Answer:
a. the kinetic energy will be the same.
b.
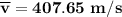
Step-by-step explanation:
The kinetic theory deals with how the arrangement of particles of a substance determines the properties that substance will possess and the state in which it is likely to be found under a given set of conditions. From the postulates of the kinetic theory of gases, The average kinetic energy of the gas molecules is a measure of the temperature of the gas molecules, This implies that the kinetic energy is dependent on the temperature.
i.e
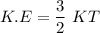
where ;
K = Boltzmann Constant
Since the given gases are in thermal equilibrium, therefore, the kinetic energy will be the same.
b).
The root mean square velocity of CO2 molecule is represented by the equation :