Answer:
(a) 3.33 mol Bi
(b) 140. g CO
Step-by-step explanation:
Step 1: Convert 775g to moles
Bi Molar Mass - 208.98 g/mol × 2 = 417.98 g/mol
O Molar Mass - 16.00 g/mol × 3 = 48.00 g/mol
775g Bi₂O₃ ÷ 465.98 g/mol = 1.66316 mol Bi₂O₃
Step 2: Find the conversion from Bi₂O₃ to Bi
1 mole of Bi₂O₃ equals 2 moles of Bi
Step 3: Use Dimensional Analysis
1.66316 mol ·
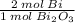 = 3.32632 mol Bi
= 3.32632 mol Bi
3.32632 mol Bi ≈ 3.33 mol Bi (3 significant figures)
Step 4: Find the conversion from Bi₂O₃ to CO
1 mole of Bi₂O₃ equals 3 moles of CO
Step 5: Use Dimensional Analysis
1.66316 mol ·
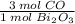 = 4.98948 mol CO
= 4.98948 mol CO
Step 6: Find molar mass of CO and convert moles to grams
C - 12.01 g/mol
O - 16.00 g/mol
4.98948 mol CO · 28.01 g/mol = 139.775 g CO
139.775 g CO ≈ 140. g CO (3 significant figures)