Given :
Half-life of Iodine ,
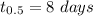 .
.
Initial concentration ,
![[A_o]=100\ mg](https://img.qammunity.org/2021/formulas/chemistry/high-school/hg1vv75zi7ahn4oqbtildbvnbs5tprgb9b.png) .
.
To Find :
The amount of sample remains after 32 days .
Solution :
Rate constant is given by :
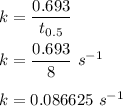
Now , by rate law :
![[A]=[A_o]e^(-kt)](https://img.qammunity.org/2021/formulas/chemistry/high-school/7euyxtrvqgqdgeckv2x13x02not8u0swjc.png)
Putting all given values, we get :
![[A]=100* e^(-(0.086625)* 32)\\](https://img.qammunity.org/2021/formulas/chemistry/high-school/z5zwgjrlm34a2eef4gaw8qr4nup1pnojya.png)
![[A]=6.25\ mg](https://img.qammunity.org/2021/formulas/chemistry/high-school/rhwc2s10p9bv47tjzl9379co1ebq7femlx.png)
Therefore , the remaining sample after 32 days is 6.25 mg .
Hence , this is the required solution .