Answer:
The temperature rises because for a given volume of gas, a rise pressure of the gas in pressure results in a proportionate rise in the temperature of the gas
Similarly when the handle is raised to draw air causes a fall in pressure that results in proportionate fall in temperature, for a given volume of gas
Step-by-step explanation:
From Gay-Lussac's law, states that the pressure of a given mass of gas is directly proportional to its Kelvin temperature, provided that the volume is held constant
Mathematically, the law states that Pressure ∝ Temperature, at constant Volume
Therefore;
P₁/T₁ = P₂/T₂
Similarly, by kinetic theory of gases, we have;
The
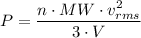
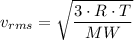
Therefore, as in order for the hand pump to inflate the bicycle tires, the air in the pump has to be compressed to force it into the tire, thereby increasing the pressure, of the air in a given volume of the pump which results in the raising of the temperature of the air in the pump, which raises the temperature of the wall of the pump.
The temperature of the air in the pump also falls as the pressure in the pump is reduced by raising the pump handle, to reduce the air pressure inside the pump and and allow air to be taken into the pump.