Answer:
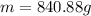
Step-by-step explanation:
Hello,
In this case, considering the ideal gas equation as:

We can first compute the moles of air at the given conditions of 195 kPa (1.92 atm), 10 °C (283K) and 350 L:
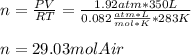
Next, since the molar mass of air is 28.97 g/mol, the mass is computed to be:
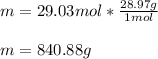
Best regards.