Answer:
c = 0.38 J/(g-°C)
Step-by-step explanation:
Given that,
Mass of copper, m = 5 g
Initial temperature,
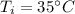
Final temperature,
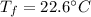
When copper cools, it looses 23.6 J of heat. We need to find the specific heat of copper. The heat lost is given by :
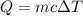
c is specific heat
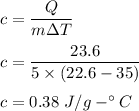
Hence, the correct option is d.