Answer:
The substance has a density of 2.636 grams per mililiter.
Step-by-step explanation:
We must remember that density (
 ), measured in grams per mililiters, is the mass of the substance (
), measured in grams per mililiters, is the mass of the substance (
 ), measured in grams, divided by its volume (
), measured in grams, divided by its volume (
 ), measured in mililiters. That is:
), measured in mililiters. That is:
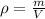
In addition, a kilogram equals 1000 grams and a liter equals 1000 mililiters. If we know that
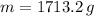 and
and
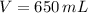 , then:
, then:
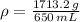
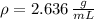
The substance has a density of 2.636 grams per mililiter.