Answer:
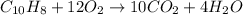
Step-by-step explanation:
Hello,
In this case, since combustion reactions yield carbon dioxide and water via the following equation:
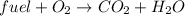
In such a way, for naphthalene, its combustion reaction is:
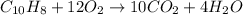
Whereas we see ten carbon atoms, eight hydrogen atoms and twenty four oxygen atoms at both reactants and products as a proof of the law conservation of mass.
Regards.