Step-by-step explanation:
The pH of a substance can be found by using the formula
![pH = - log[ H^( + ) ]](https://img.qammunity.org/2021/formulas/chemistry/high-school/77ce7svtu6hkt9nopiaycl1bg1b35anr8l.png)
where [ H+ ] is the hydrogen ion concentration in the substance
From the question
[ H+ ] = 2.1 × 10-¹² M
To find the pH substitute the value into the above formula
We have
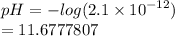
We have the answer as
pH = 11.7
The solution is basic since it lies in the basic region that is from 8 to 14
Hope this helps you