Answer:
Most similar ----- Lithium
Least similar ---- Nitrogen
Step-by-step explanation:
Cesium is an element on the periodic with the atomic number 133. It lies in group 1 (i.e., the alkali metals) and period 6 on the periodic table. The oxidation state of group 1 metals is +1. Cesium forms an oxide with oxygen as
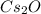 .
.
The most similar compound to this chemical compound is Lithium because Lithium happens to be in the same group one metal with Cesium and forms the compound
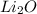 with the oxygen
with the oxygen
The least similar compound nitrogen due to fact that it is an oxide that is covalent in nature and lies between-group 3 -17 to form an
 with oxygen.
with oxygen.