Complete Question
Which is most reactive toward electrophilic aromatic substitution?
A Phenol
B Anisole
C Nitro-benzene
D Benzene
Answer:
The correct option is A
Step-by-step explanation:
Generally the rate at which electrophilic aromatic substitution reaction occur is dependent on the nucleophilicity(nucleophile strength ) of Benzene ring.Now a functional group which increase the electron density in the benzene ring when attached to it increases its nucleophilicity and this in-turn will increase the rate at which electrophilic aromatic substitution reaction occur
Now Phenol is a compound that is made up of OH functional group and then the benzene ring
Anisole is a compound that is made up of
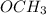 functional group and then the benzene ring
functional group and then the benzene ring
Nitro-benzene is a compound that is made up of
 functional group and then the benzene ring
functional group and then the benzene ring
Now since OH is the highest In series of activating group compared to the activation groups we are considering (i.e
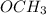 and
and
 )
)
Then Phenol is the answer