Answer:
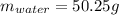
Step-by-step explanation:
Hello,
In this case, considering that the by-mass percent of water is:
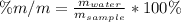
Given such percent and the mass of the sample, we can find the mass of water in grams in the sample by solving for it as shown below:
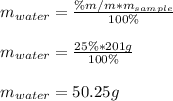
Best regards.