Answer:
0.11 M
Step-by-step explanation:
The computation of molarity of the CH3COOH solution is shown below:-
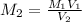
Where,
 = Molarity of LioH = 0.0175
= Molarity of LioH = 0.0175
 = Volume of LioH = 18.5 ml
= Volume of LioH = 18.5 ml
 = Volume of CH3COOH = 29.4 ml
= Volume of CH3COOH = 29.4 ml
Now, we will put the values into the formula
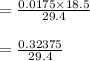
Which gives result
= 0.011 M
Therefore for computing the molarity of the CH3COOH solution we simply applied the above formula.