Answer:
The molar mass of the gas should be 43.0 g/mol.
Step-by-step explanation:
1.Use the ideal gas law(
 ) & the formula for moles and molar mass(
) & the formula for moles and molar mass(
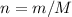 ).
).
2.Insert the equation
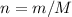 into
into
 and re-arrange to solve for M(molar mass).
and re-arrange to solve for M(molar mass).
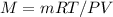
3.Insert values and use the gas constant R =
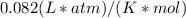 since the volume is in liters and the pressure is in atmosphere units.
since the volume is in liters and the pressure is in atmosphere units.
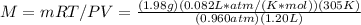
4. Your answer should be 42.99 g/mol or 43.0 g/mol.
Have fun with chemistry!