Answer:
Formic acid and Acetic acid is the best buffer at pH 3.7.
Step-by-step explanation:
Given that,
The Ka values for several weak acids are given,
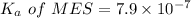
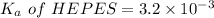
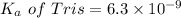
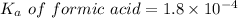
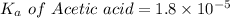
We need to calculate the pH of the weak acids with their
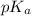 values
values
Using formula of
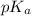
For MES,
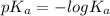
Put the value into the formula
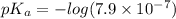
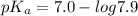
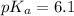
pH range for best buffer,
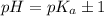
Put the value into the formula
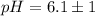
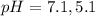
The pH value of the solution between 7.1 to 5.1.
This is not best buffer.
For HEPES,
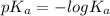
Put the value into the formula
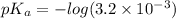
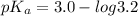
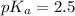
pH range for best buffer,
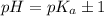
Put the value into the formula
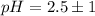
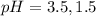
The pH value of the solution between 3.5 to 1.5.
This is not best buffer.
For Tris,
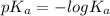
Put the value into the formula
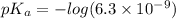
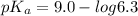
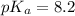
pH range for best buffer,
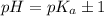
Put the value into the formula
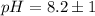
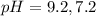
The pH value of the solution between 9.2 to 7.2.
This is not best buffer.
For formic acid,
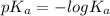
Put the value into the formula
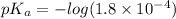
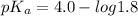
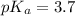
pH range for best buffer,
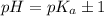
Put the value into the formula
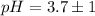
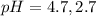
The pH value of the solution between 4.7 to 2.7.
This is best buffer.
For acetic acid,
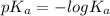
Put the value into the formula
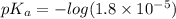
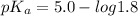
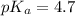
pH range for best buffer,
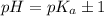
Put the value into the formula
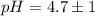
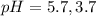
The pH value of the solution between 5.7 to 3.7.
This is best buffer
Hence, Formic acid and Acetic acid is the best buffer at pH 3.7.