Answer:
Mass of Iron is 24.45 kg
Step-by-step explanation:
Given that:
Mass of Silver,
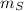 = 8.00 kg
= 8.00 kg
Mass of Gold,
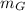 = 15.0 kg
= 15.0 kg
Initial temperature of Silver and Gold = 333 K
Initial temperature of Iron = 1737 K
Final temperature = 1337 K
Specific heat capacity of Gold-liquid,
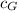 = 0.150 kJ/(kg K)
= 0.150 kJ/(kg K)
Specific heat of Silver-liquid,
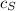 = 0.280 kJ/(kg K)
= 0.280 kJ/(kg K)
Known: Specific heat capacity of Iron,
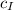 = 0.461 kJ/(kg K)
= 0.461 kJ/(kg K)
Therefore;
Heat lost by Iron = Heat gained by Silver + Heat gained by Gold
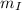 x
x
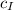 x Δθ = (
x Δθ = (
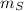 x
x
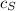 +
+
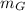 x
x
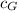 ) x Δθ
) x Δθ
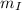 x 0.461 x (1737 - 1337) = (8 x 0.280 + 15 x 0.150) x (1337 - 333)
x 0.461 x (1737 - 1337) = (8 x 0.280 + 15 x 0.150) x (1337 - 333)
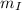 x 0.461 x 400 = (2.24 + 2.25) x 1004
x 0.461 x 400 = (2.24 + 2.25) x 1004
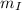 x 184.4 = 4507.96
x 184.4 = 4507.96
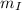 =
=
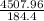
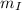 = 24.4466
= 24.4466
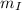 ≅ 24.45
≅ 24.45
The mass of Iron is 24.45 kg.