Answer:
It takes 1,068.76 grams of nitrogen to fill an 855 L tank at STP.
Step-by-step explanation:
The STP conditions refer to the standard temperature and pressure. Pressure values at 1 atmosphere and temperature at 0 ° C or 273.15 °K are used and are reference values for gases.
On the other side, the pressure, P, the temperature, T, and the volume, V, of an ideal gas, are related by a simple formula called the ideal gas law:
P*V = n*R*T
where P is the gas pressure, V is the volume that occupies, T is its temperature, R is the ideal gas constant, and n is the number of moles of the gas.
So, in this case:
- P= 1 atm
- V= 855 L
- n= ?
- R= 0.082
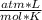
- T= 273.15 K
Replacing:
1 atm* 855 L= n* 0.082
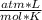 * 273.15 K
* 273.15 K
Solving:
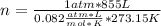
n= 38.17 moles
Being the molar mass of nitrogen N2 equal to 28 g / mol, you can apply the following rule of three: if there are 28 grams in 1 mole, how much mass is there in 38.17 moles?
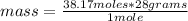
mass= 1,068.76 grams
It takes 1,068.76 grams of nitrogen to fill an 855 L tank at STP.