Answer:
It is already balanced. So, the answer is
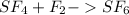
Step-by-step explanation:
In the equation on the reactant side there is 1 sulfur atom, this is the same on the other side of the equation as well (products).
There are a total of 6 fluorine atoms on the reactant side once the 4 and 6 are added together. Then, in the products, there are 6 fluorine atoms as well.
Thus, the equation is already balanced.
Hope this helps!! Ask questions if you need!