Answer:
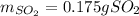
Step-by-step explanation:
Hello,
In this case, since the first step is to write the properly balanced chemical reaction:
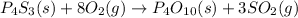
We can see that given the 0.200 g of P4S3 (molar mass 220 g/mol) the mole ratio between it and SO2 (molar mass 64 g/mol) is 1:3, therefore, the produced mass of SO2 turns out:
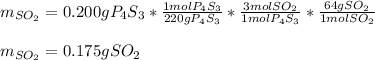
Best regards.