Answer:
pH = 5.9
Step-by-step explanation:
The equation to find pH is as follows:
pH = -log[H+]
We are not given [H+] so we have to find it given the value of [OH-].
To find [H+] from [OH-], use this equation:
![[H^(+) ]=(1.0*10^(-14) )/([OH^(-)] )](https://img.qammunity.org/2023/formulas/chemistry/high-school/raeggwek43jvflcmkiad51kdytxy744bfl.png)
Plug in [OH-] and solve for [H+]:
![[H^(+) ]=(1.0*10^(-14) )/(7.1*10^(-9) )](https://img.qammunity.org/2023/formulas/chemistry/high-school/r0p1ndse50d7xqjfnoy4a8yarvado5qprf.png)
[H+] =
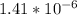
Now that we have found [H+] plug it into the pH equation and solve:
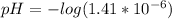
pH = 5.9
So, the pH of the solution would be 5.9
Hope this helps!! Ask questions if you need!