Answer:
There are
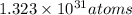 of mercury in 4,408 metric tons.
of mercury in 4,408 metric tons.
Step-by-step explanation:
Mass of mercury metal = 4,408 metric ton
1 metric ton = 1000 kg
4,408 metric ton = 4,408 × 1000 kg = 4,408,000 kg
1 kg = 1000 g
4,408,000 kg = 4,408,000 × 1000 g = 4,408,000,000 g
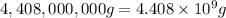
Mass of mercury =
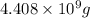
Atomic mass of mercury = 200.59 g/mol
Moles of mercury = n
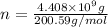
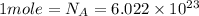 atoms/molecules/ ions
atoms/molecules/ ions
Number of atoms of mercury in n moles = N
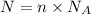
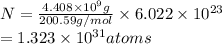
Hence, there are
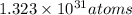 of mercury in 4,408 metric tons.
of mercury in 4,408 metric tons.