Given :
Mass of oxygen containing carbon monoxide (CO) is 2.666 gram .
To Find :
How many grams of carbon (C) would be present in carbon monoxide (CO) that contains 2.666 grams of oxygen (O) .
Solution :
By law of constant composition , a given chemical compound always contains its component elements in fixed ratio (by mass) and does not depend on its source and method of preparation.
So , volume of solution does not matter .
Moles of oxygen ,
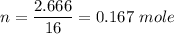 .
.
Now , molecule of CO contains 1 mole of C .
So , moles of C is also 0.167 mole .
Mass of carbon ,
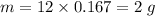 .
.
Therefore , mass of carbon is 2 grams .
Hence , this is the required solution .