Answer:
The ice occupies a volume of 1.196 liters at -10 ºC.
Step-by-step explanation:
We must remember that density (
 ), measured in grams per cubic centimeters, is the ratio of mass (
), measured in grams per cubic centimeters, is the ratio of mass (
 ), measured in grams, to occupied volume (
), measured in grams, to occupied volume (
 ), measured in cubic centimeters, that is:
), measured in cubic centimeters, that is:
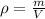
We clear the mass within the formula:
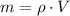
The mass of the water inside the soft-drink bottle is: (
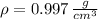 and
and
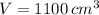 )
)
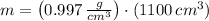
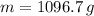
There are 1096.7 grams of water filling the soft-drink bottle completely.
Then, the water is frozen to -10 ºC and transformed into ice, the volume occupied by the ice which we can deduct from definition of density. That is:
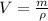
The volume occupied by the ice inside the soft-drink bottle is: (
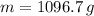 and
and
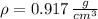 )
)
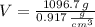
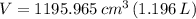
The ice occupies a volume of 1.196 liters at -10 ºC.