Answer:
the new volume is 1.35L
Step-by-step explanation:
The computation of the new volume in the case when the temperature rises to 45 degrees is shown below:
GIven that
Volume = 1.25L
And, the temperature is 22 degrees celsius
So,
 = (275 + 22)k
= (275 + 22)k
= 297 k
Now
 = (275 + 45)k
= (275 + 45)k
= 320 k
Now as it is a constant pressure
So, the new volume is
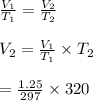
= 1.35L
hence, the new volume is 1.35L