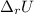Answer:
The correct option is C
Step-by-step explanation:
From the question we are told that
The reaction is
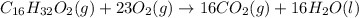
Generally
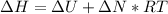
Here
 is the change in enthalpy
is the change in enthalpy
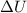 is the change in the internal energy
is the change in the internal energy
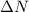 is the difference between that number of moles of product and the number of moles of reactant
is the difference between that number of moles of product and the number of moles of reactant
Looking at the reaction we can discover that the elements that was consumed and the element that was formed is
 and
and
 and this are both gases so the change would occur in the number of moles
and this are both gases so the change would occur in the number of moles
So
![\Delta H = \Delta U + [16 -23]* RT](https://img.qammunity.org/2021/formulas/chemistry/college/d94cvt0j2wpx5b91ph2awmxvvpe1biruwo.png)
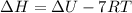
The negative sign in the equation tell us that the enthalpy
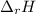 would be less than the Internal energy
would be less than the Internal energy