Answer:
482.5 grams
Step-by-step explanation:
Given that,
Volume of a cube is 25 mL
The density of gold is 19.3 g/mL
We need to find the mass of the gold cube. We know that density is equal to the mass per unit volume. So,
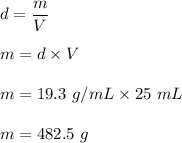
So, the mass of the gold cube is 482.5 grams.