Answer:
a.
![r=k[B]^(1/2)[C]^2](https://img.qammunity.org/2021/formulas/chemistry/college/uq8mf0izj8uqenvfw107tbh4myubge0pyp.png)
b.
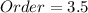
c. It does not affect the rate.
d.
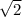 .
.
e. 4.
f. 4
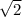 .
.
Step-by-step explanation:
Hello,
In this case, considering the given information, we have:
a.
![r=k[B]^(1/2)[C]^2](https://img.qammunity.org/2021/formulas/chemistry/college/uq8mf0izj8uqenvfw107tbh4myubge0pyp.png)
b. By adding 1/2 and 2 (powers for B and C), the overall order is:
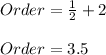
c. It is not changed, since the concentration of A is not affecting the rate due to its specific zeroth-order.
d. In this case, if the concentration of B is doubled, such term in the equation shows:
![\sqrt[n]{x} (r_f)/(r_i)=([2B]^((1/2)) [C]^2)/([B]^((1/2))[C]^2) \\\\(r_f)/(r_i)=([2B]^((1/2)))/([B]^((1/2)))\\\\(r_f)/(r_i)=((2)/(1))^(1/2)\\\\r_f=√(2) r_i](https://img.qammunity.org/2021/formulas/chemistry/college/ae6qzxgsys4z3bobwvna7ggmure5mdpj5s.png)
It means that the rate increases by a factor of
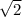 .
.
e. In this case, if the concentration of C is doubled, such term in the equation shows:
![(r_f)/(r_i)=([B]^((1/2))[2C]^2)/([B]^((1/2))[C]^2) \\\\(r_f)/(r_i)=(([2C])/([C]))^(2)\\\\(r_f)/(r_i)=((2)/(1))^(2)\\\\r_f=4r_i](https://img.qammunity.org/2021/formulas/chemistry/college/3f83c66s33k8u88dhjzan3qww5boz3v9no.png)
It means that the rate increases by a factor of 4.
f. In this case, if the concentration of both B and C are doubled, such terms in the equation shows:
![(r_f)/(r_i)=([2B]^((1/2))[2C]^2)/([B]^((1/2))[C]^2) \\\\(r_f)/(r_i)=(2^((1/2))2^2)/(1^((1/2))1^2) \\\\(r_f)/(r_i)=4√(2) \\\\r_f=4√(2) r_i](https://img.qammunity.org/2021/formulas/chemistry/college/e14uxab47sricipta1qenwi6gegyh5bf0l.png)
It means that the rate increases by a factor of 4
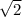 .
.
Best regards.